The La Torre Lab studies how the central nervous system generates its remarkable variety of neurons during development. The resulting organization is just extraordinary: billions of neurons, encompassing thousands of distinct cell types, assemble into layered and interconnected networks with exquisite precision to control every aspect of our brain functions, from vision and memory to emotion and behavior.
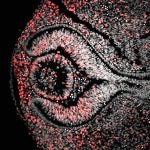
We study two parts of the central nervous system that illustrate this complexity: the retina, which turns light into vision, and the neocortex, which makes sense of the world and directs movement. In both tissues, development begins with a simple sheet of cells called neuroepithelium. Over time, the neuroepithelium transforms into a highly organized, layered structure packed with specialized neurons that connect with remarkable precision to form functional circuits.
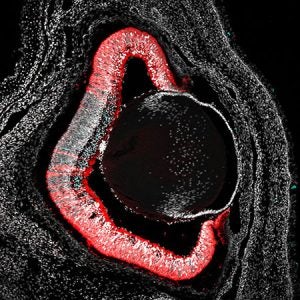
In the developing retina, the transformation of a simple neuroepithelial sheet into a complex, layered tissue begins very early during embryogenesis. This process begins with the evagination of the optic vesicles from the neural tube, which then invaginate to form the optic cup, establishing the foundational architecture of the eye. These morphogenetic events are orchestrated by a combination of intrinsic transcriptional programs and extrinsic signaling cues, including morphogens such as Sonic Hedgehog (Shh), Fibroblast Growth Factors (FGFs), Bone Morphogenetic Proteins (BMPs), and Wnts. Within the retina, the retinal progenitor cells -the stem cells that give rise to all retinal neurons- follow a carefully timed temporal program, in which their competence to produce specific neuronal subtypes changes over developmental time. This ensures that neurons are generated in the correct order and at the appropriate stage, laying the foundation for a functional retinal circuit.
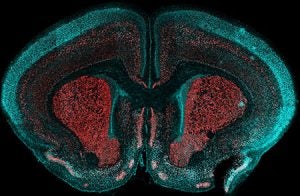
A parallel process occurs in the neocortex, where cortical progenitors also exhibit temporal competence, producing distinct neuronal subtypes in a sequence. Following their birth, these neurons migrate radially to their appropriate cortical layers, where they integrate into functional circuits. Together, the temporally regulated output of progenitors in both the retina and neocortex drives the formation of layered and interconnected networks, generating the cellular diversity and circuit architecture necessary for normal function.
Our lab investigates the mechanisms that orchestrate morphogenesis and progenitor competence, with a particular focus on microRNAs (miRNAs), small non-coding RNAs that fine-tune gene expression and coordinate the timing of cell cycle exit and fate specification. Using in vivo models, stem cell–derived organoids, and targeted molecular perturbations, we aim to uncover how spatial patterning cues, temporal programs, and post-transcriptional regulation converge to generate the extraordinary cellular diversity of the retina and neocortex.
Curious about the details? Explore our work:
- Krueger et al.: Novel Roles of Sonic Hedgehog Signaling in Retinal Patterning and Neurogenesis During Mammalian Eye Development.
- Decker et al.: Uncoupling Neocortical Neuron Fate and Migration via a Let-7–RBX2 Axis.
- Han et al, 2023. Development. Notch directs telencephalic development and controls neocortical neuron fate determination by regulating microRNA levels.
- Fishman et al. 2022. Front. Cell. Dev. Biol. Oscillatory Behaviors of microRNA Networks: Emerging Roles in Retinal Development.
- Fairchild et al, 2019. Sci. Rep. Let-7 regulates cell cycle dynamics in the developing cerebral cortex and retina.
- La Torre et al. 2013. PNAS. Conserved microRNA pathway regulates developmental timing of retinal neurogenesis.
